BASICS
A fuel cell is a device, composed of an anode, an electrolyte membrane, and a cathode, that generates electricity through an electrochemical reaction. First, hydrogen is passed through the anode which causes the hydrogen molecules to split. The molecules are split into electrons and protons. The electrons are pushed through a circuit, generating electricty. The protons pass through the electrolyte membrane. Oxygen is passed through the cathode where it is combined with the electrons and protons to produce the fuel cell’s byproducts: water and heat.
There are several types of fuel cells. A few are listed below. Basic operation of all fuel cells are relatively similar. For more information regarding these different types of fuel cells, please visit:https://www.energy.gov/eere/fuelcells/fuel-cell-basics
|
|
BENEFITS
Fuel cells do not have any moving parts causing them to operate almost silently.
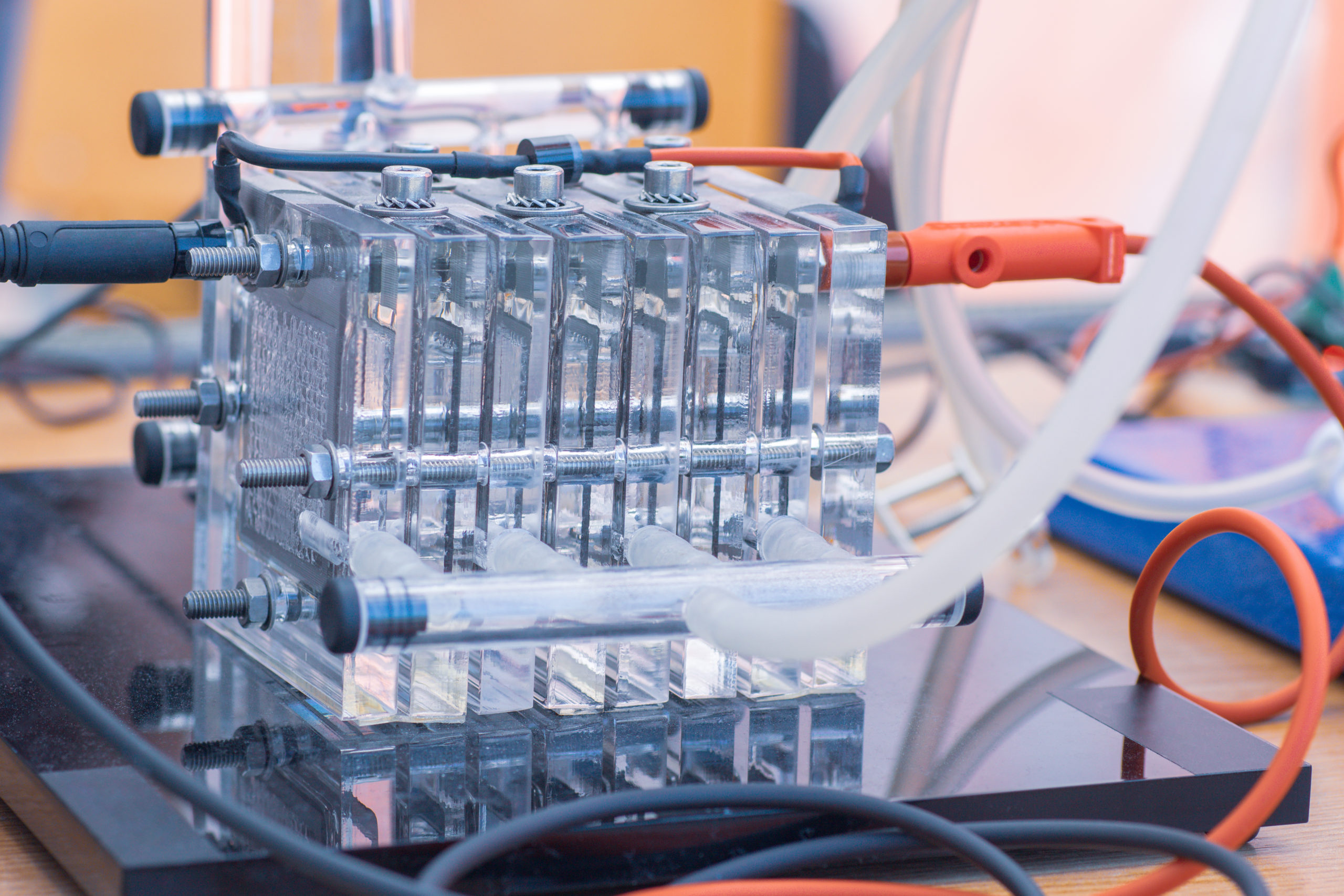
Fuel cells are scalable therefore they can range in size allowing them to provide heat and electricity for homes and businesses as well as electrical power for vehicles and electronic devices.
Unlike batteries, fuel cells do not need to be recharged and can run indefinitely, as long as hydrogen and oxygen are supplied. Fuel cells have proven to be an extremely efficient, reliable, and durable source of power, as witnessed by their extensive use in the NASA and other space programs starting with the Gemini vessels.
Today, their application has been extended to stationary uses such as auxiliary or primary power supply, CHP systems, and mobile uses for electronics or light to heavy duty vehicles.
Fuel cell technology coupled with Ways2H systems are pathways for a cleaner environment. Together our technologies are inherently clean and reliable providing predictable, on-deman hydrogen-based power.
http://www.fchea.org/fuelcells
http://www.zeroemissions.org/wp-content/uploads/2016/10/FCHEA_Fuel-Cells-101_Factsheet-1.pdf
https://www.energy.gov/eere/fuelcells/fuel-cell-basics